In the Philippines, 75 Filipinos die of tuberculosis every day, and it is the 6th leading cause of mortality in the country. The Philippines ranks eighth among 22 countries with the highest burden of tuberculosis.
Here is the current TB situation in the Philippines:
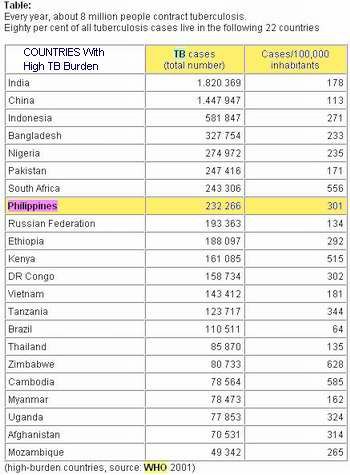
TB Rank: 8th among 22 countries
In spite of the depressing scenario of our TB situation, the World Health Organization (WHO) has noted optimistically that the tuberculosis situation in the Philippines is improving as 164 patients get treatment every day, or around 60,000 of the 65,000 new cases in 2002.
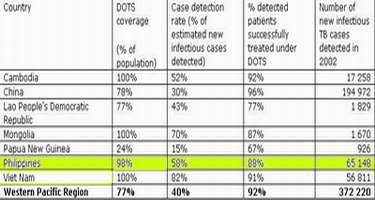
Good DOTS Coverage and Treatment
For those not familiar with TB treatment, DOTS means directly-observed treatment short course. It is a treatment strategy for tuberculosis promoted by WHO that involves having someone supervising the TB patient's timely intake of his/her TB medicines. This person can be a health personnel or one of the patient's relatives.
The biggest stumbling blocks in the war against TB here and abroad have been the development of resistant TB strains, cases of re-infection, and non-compliance or difficulty in taking the prescribed treatment course. DOTS has addressed the last problem quite suceesfully as can be seen from Table II above.
The good news for TB patients recently is the development of a new antibiotic class for killing Koch's bacilli:
This is very, very good news especially for Filipino TB patients.WASHINGTON (Reuters) --- A novel antibiotic that is working well in mice may be the first new drug enlisted in the fight against tuberculosis in 40 years, Johnson & Johnson said on Thursday.
It works in a completely new way to shut down the bacteria that causes tuberculosis, said Dr. Koen Andries of the U.S. drug and household products giant, who led the study.
"This drug is apparently cutting off the energy supply of the bacteria," Andries said in a telephone briefing.
It might be added to the cocktails used for tuberculosis to speed up the treatment, which currently takes months, the researchers said.
Originally developed as an immune system drug, the new drug was a failure and languished in the drug library of Johnson & Johnson before being rediscovered, Andries said.
The drug, known only by its experimental name R207910, has been tested in people in a Phase I safety trial and seems to cause no serious side effects. The company is proceeding to Phase II, the second of the three phases of experiments needed before seeking U.S. Food and Drug Administration approval.
The drug is in a new class called DIARYLQUINOLINES
It works by shutting down the bacteria's source of power, an enzyme called ATP synthase.
World Health Organization tuberculosis expert Dr. Christopher Dye called the finding a breakthrough.
[Reuters Health]
We have long been tormented by this dreadful disease. It is about time we show those tubercle bacilli a new weapon in the armory.
0 reactions:
Post a Comment